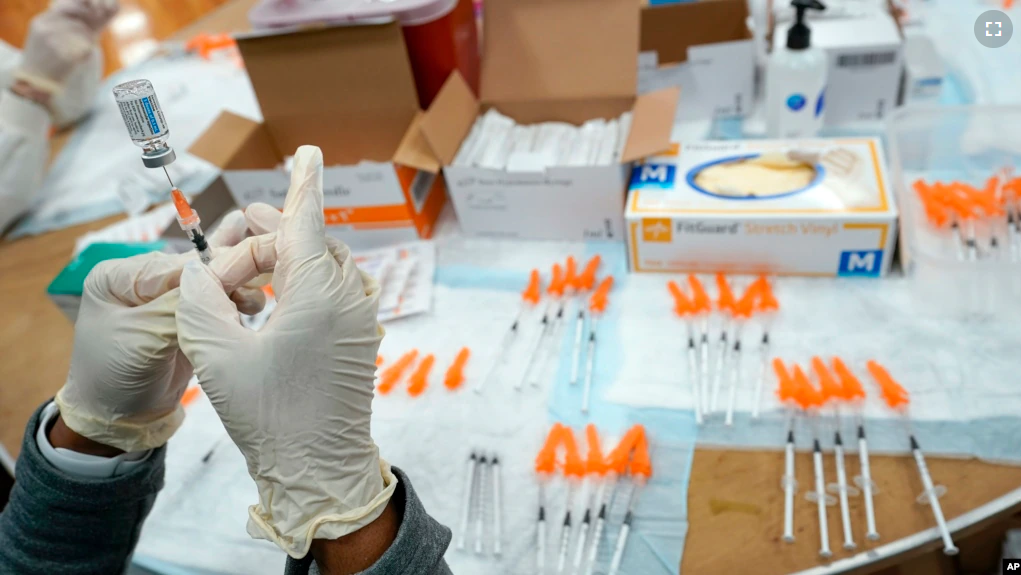
The U.S. Food and Drug Administration, or FDA, says COVID-19 booster shots for autumn will have to add protection against the latest Omicron sub-variants.
Two widely used COVID-19 shots made by Moderna and Pfizer-BioNTech have saved millions of lives worldwide in just their first year of use.
The FDA said they would be used for anyone still getting their first shots. But with immunity waning and the super-contagious Omicron family of variants getting better at escaping antibodies protection, the boosters will need an update. Waning immunity means that the effectiveness of the vaccine decreases over time.
Fast spreading variants
Antibodies are the first layer of defense that form after vaccination or an infection. They can prevent infection by recognizing the outer coating of the new coronavirus — the spike protein — and blocking it from entering your cells.
Separate studies published recently in Nature and the New England Journal of Medicine show the newest sub-variants of Omicron are even better at avoiding antibodies. The studies showed this happened both in the vaccinated and in people who recovered from the original Omicron variant.
Drug companies Moderna and Pfizer said they were already working on booster shots that add protection against Omicron. The new combination shots, called “bivalent” by scientists, showed increased levels of antibodies against the original Omicron variant. But they did not provide nearly as many antibodies against its two sub-variants, known as BA.4 and BA.5. Scientists support the “bivalent” shot because it combines the original vaccines’ proven benefits with new protection against other variants.
The Centers for Disease Control and Prevention (CDC) said the two worrisome sub-variants now make up half of all infections in the U.S. Both Moderna and Pfizer said they will have combination shots to deal with Omicron by October.
A Pfizer spokeswoman told the Associated Press in an email: “We’re continuing to collect more data from our study on BA.4/5 and will be in touch as soon as we are ready to submit.” But Moderna said changing the shots to target the sub-variants might delay its version another month.
A third company, Novavax, is awaiting FDA authorization for its more traditional, protein-based COVID-19 vaccine. Novavax argued that a booster of its regular vaccine promises higher immunity against the Omicron variant.
Decision time for the FDA
“None of us has a crystal ball” to know the next threatening variant, said FDA vaccine chief Dr. Peter Marks last week. The expression having a crystal ball means having the ability to predict the future. Marks added that the agency will try to “bring the immune system closer” to the new variants rather than the older ones.
“We would obviously like to get it right enough [so that with one more shot] we get a full season of protection,” he said.
The FDA’s order does not guarantee that those combination shots would be offered in autumn. Drug makers still must provide data from human trials before the agency decides whether to authorize the modified boosters. Then, the CDC would have to decide how they are used.
It is not clear who would be offered a new combination booster shot. They might be urged only for older adults or those at high risk from the virus.
Advisers to the World Health Organization (WHO) recently said Omicron-targeted shots would be most useful as a booster because they should increase cross-protection against several variants.
Dr. Kanta Subbarao is a virologist who heads the WHO committee. “We don’t want the world to lose confidence in vaccines that are currently available,” she said.
I’m Jonathan Evans.
Lauran Neergaard and Matthew Perrone reported on this story for the Associated Press. Jonathan Evans adapted this story for Learning English.
_____________________________________________________________________
Quiz – US: COVID Shots for Autumn Must Target Omicron
Start the Quiz to find out
_____________________________________________________________________
Words in This Story
booster – n. a substance or dose used to renew or increase the effect of a drug or immunizing agent
contagious – adj. transmissible by direct or indirect contact with an infected person
authorization – n. the act of authorizing
confidence – n. the quality or state of being certain